Des gamètes à la naissance : génomique, épigénétique et physiopathologie de la reproduction
Responsable(s)
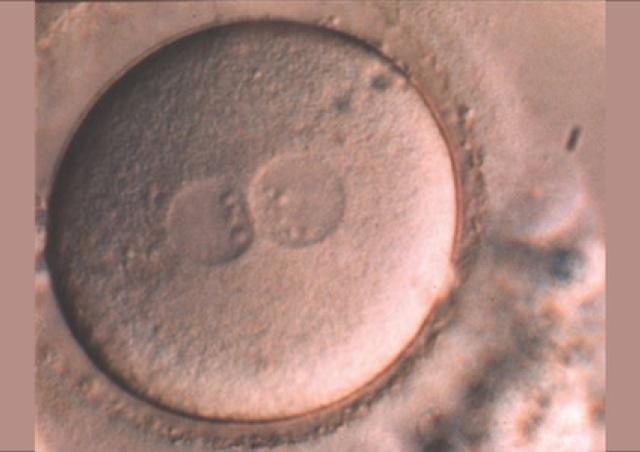
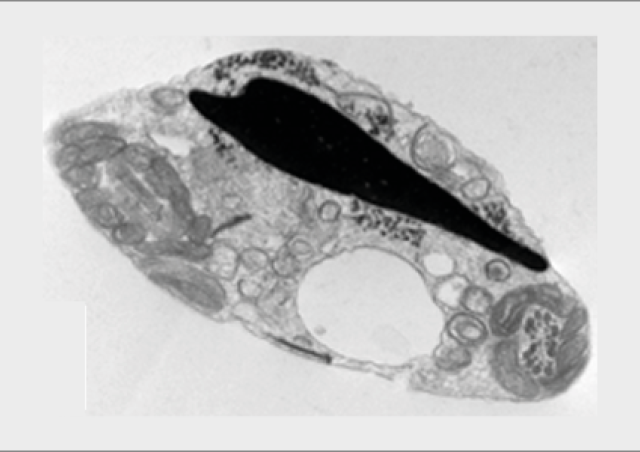
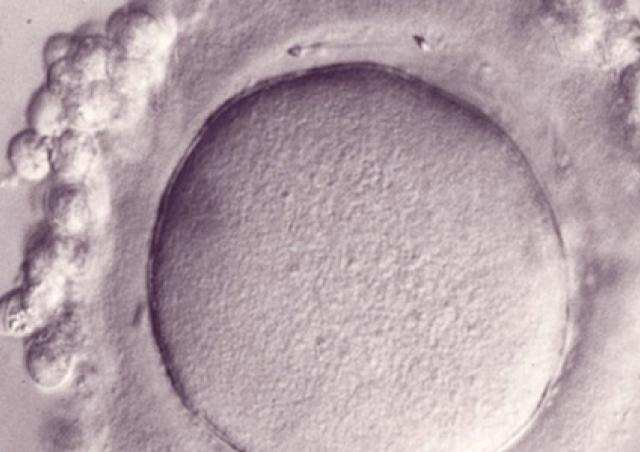
L’équipe "From Gametes To Birth" vise à étudier les désordres de la fonction reproductive humaine allant de l’infertilité aux pathologies de la grossesse. Parmi les maladies clefs qui sous-tendent nos questions se trouvent les défauts de différenciation des gamètes mâles, les défauts d’interaction spermatozoïde-ovocyte, les défauts d’implantation ou de fonctionnement utérin (en particulier lors de l’endométriose), les maladies hypertensives de la grossesse (prééclampsie) et les anomalies de la durée de la grossesse, comme l‘accouchement prématuré. En outre, nous consacrons une part de notre activité de recherche à l’impact des perturbations environnementales sur les fonctions de reproduction. Pour finir, nous étudions également les conséquences à long terme de perturbations de la gamétogenèse, de la placentation, qu’elles soient dues à l’environnement ou à une pathologie.
Notre équipe étudie les mécanismes physiopathologiques et moléculaires sous-jacents aux défauts de la reproduction par une approche intégrée à l’interface entre recherche fondamentale et clinique. Notre recherche implique l’étude de la différenciation cellulaire, la régulation de l’expression des gènes, le développement, la fusion cellulaire et l’immunologie.
Nos approches méthodologiques font appel à la biologie cellulaire et moléculaire, la microscopie optique et électronique, la génétique (contextes familiaux ou populationnels), les techniques de biologie de la reproduction, ainsi que les approches à haut-débit «omics» telles que la génomique, la transcriptomique, la protéomique et les analyses bioinformatiques de ces données. Ces dernières années, nous avons en particulier mis en place des approches de séquençage d’ARN sur cellules isolées issues des membranes fœtales et du placenta. Afin de produire les modèles les plus pertinents, nous utilisons des approches d’édition du génome dans des cellules et dans des modèles murins, et nous collaborons à des approches de développement d’organoïdes.