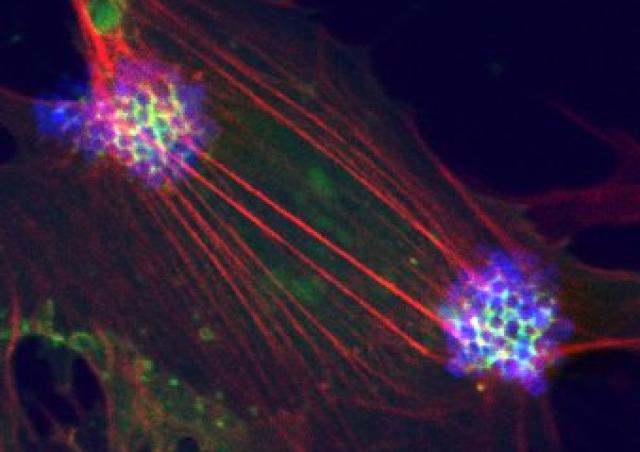
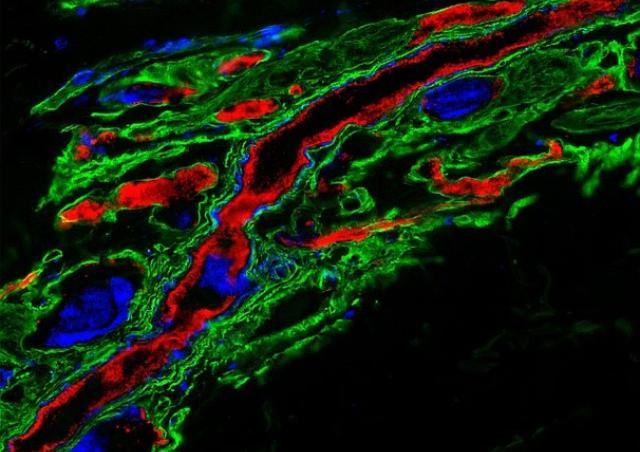
Inhibition of the tyrosine kinase receptor ErbB2/HER2: from bacterial meningitis to human cancers
Project leader(s)
Among the ErbB family of tyrosine kinase receptors, ErbB2/HER2 displays unique properties. This orphan receptor exists in a ligand-independent, activated configuration that promotes cell transformation when overexpressed. Intriguingly, this structure does not lead readily to ErbB2 activation. We previously showed that infection of endothelial cells by N. meningitidis induces the selective recruitment and activation of ErbB2 (1). Taking advantage of this host-pathogen interaction which constitutes a unique tool to study the mechanisms of ErbB2 activation, we identified a novel protein promoting the allosteric inhibition of the ligand-independent activation of ErbB2 (Patent WO/2011/036211), and which is repressed in HER2+ breast cancers (PCT/EP2018/079213). Based on this interaction, we set up a high throughput screening method (Patent FR 14 52246) and identified novel molecules with potent anti-HER2 activity that mimic the suppressor activity (Patent WO/2017/121755). These molecules, able to cross the blood-brain barrier, inhibit both the progression of human breast cancer overexpressing HER2 and brain tumor progression (2).
Now, we aim at elucidating the mechanisms regulating gene expression of the allosteric inhibitor in HER2+ breast cancers with a focus on the role of miRNAs, key regulators of gene expression often deregulated in cancers. We identified key miRNAs representing good diagnosis and prognosis biomarkers for HER2+ breast cancers (Patent PCT/EP2018/079215) and showed that their inhibition can efficiently repress HER2 activation/expression in breast cancer tumors (Patent PCT/EP2018/079212). Our current objective is to identify the direct targets of these miRs, that contribute to these inhibitory effects. These approaches represent innovative complementary strategies for HER2+ breast cancer therapies.
1. (Hoffmann et al., J Cell Biol 2001) 2. (Faure et al., Cancer Res 2021).