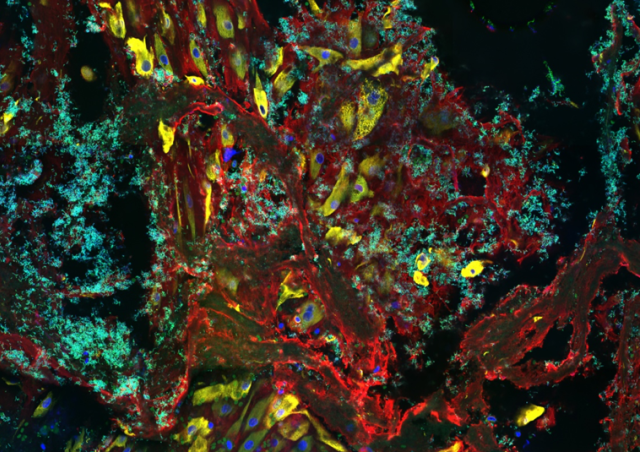
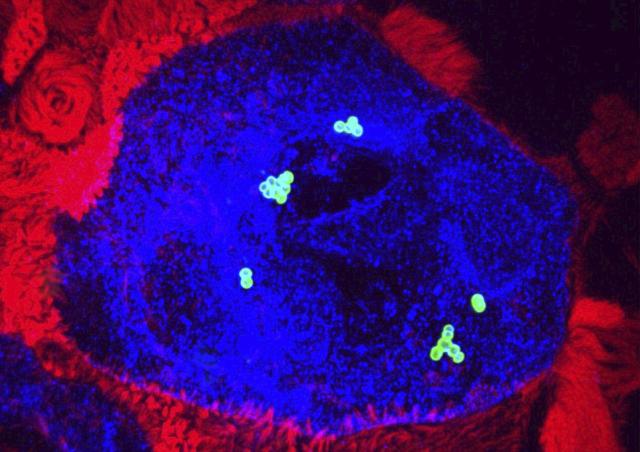
Members of the project
Project
Group B Streptococcus (Streptococcus agalactiae, GBS) is a commensal of the gastrointestinal and vaginal tracts found in 10 to 30% of healthy individuals. This Gram-positive encapsulated bacterium is, nevertheless, a deadly pathogen for newborns since it represents the main agent responsible for invasive neonatal infections (bacteremia, meningitis). Despite considerable progress in patient management, GBS meningitis remains a public health problem with a mortality rate of 10 to 20% and neurological sequelae present in 25 to 50% of surviving newborns. Worldwide epidemiological data have identified that GBS strains responsible for human infections are distributed among five major clonal complexes (CC), one of which has been designated as hypervirulent (CC17 clone) as it is responsible for approximately 80% of neonatal GBS meningitis cases. GBS CC17 expresses specific surface proteins that confer a particular meningeal tropism.
The critical step in the development of meningitis is the transmigration of GBS from the bloodstream toward to the central nervous system (CNS). The brain is normally protected by physiological barriers that separate the blood from the brain parenchyma or cerebrospinal fluid. To invade the central nervous system and cause meningitis, bloodborne GBS CC17 must cross brain barriers. This occurs after an initial interaction between GBS CC17 and endothelial cells or epithelial cells constituting the blood-brain or blood-cerebrospinal fluid barrier, respectively. After trafficking across brain barriers, GBS CC17 may interact with cell types present in the CNS in particular neurons and glial cells.
Our project focuses on host-pathogen interactions during the development of GBS meningitis.
Our project is divided into two complementary axes. On the one hand, we are researching the mechanisms involved in the crossing of cerebral barriers and, on the other hand, we are interested in the role of glial cells in the development of GBS meningitis.

1- Crossing the brain barriers
Pathogens causing meningitis can use three non-exclusive mechanisms to cross brain barriers. A transcellular passage in which bacteria are internalized in cerebral endothelial cells or choroid plexus epithelial cells after recognition of specific cell receptor(s); a paracellular passage due to the disorganization of tight junctions which allows the passage of pathogens between cells; a passage facilitated by phagocytic cells called Trojan horse mechanism involving the transmigration of an infected phagocyte. Our team has shown that CC17 strains exhibit higher adhesion properties to brain endothelial cells than non-CC17 strains. Two CC17 GBS-specific surface adhesins, Srr2 and HvgA, account for the increased binding of CC17 GBS to brain endothelial cells compared to non-CC17 strains and contribute to BBB crossing in an in vivo meningitis model. We recently identified two cellular receptors, integrins α5β1 and αvβ3, as ligands for Srr2 adhesin that contribute to blood-brain barrier crossing. These two integrins are overexpressed in brain vessels during the post-natal period providing a first molecular explanation for the susceptibility of newborns to GBS CC17 meningitis. Our research aims at deciphering, at the cellular and molecular levels how GBS CC17 penetrates the brain by investigating the anatomical routes of infection. We also aim at deciphering by which of the three putative mechanisms of infection (paracellular, transcellular or Trojan horse mechanisms) is involved upon GBS CC17 brain invasion.
2- Role of glial cells in neonatal GBS meningitis
While we have identified bacterial ligands of endothelial cells and their endothelial cell receptors, very little is known regarding the role of CNS cells in the development of GBS meningitis. Glial cells are the most abundant cells of the CNS. Invasion of the CNS by a pathogen activates the brain innate immune system. Although the overall response is initiated to protect the CNS, recent evidence has shown that in a variety of neurological disorders, including bacterial meningitis, neuroinflammation has a deleterious effect and is the major responsible for CNS insults
Astrocytes and microglia cells are key players in brain immunity. Astrocytes also play a major role in the regulation of cerebral vascular functions by covering almost all cerebral blood vessels. Our objective is to identify the cellular and molecular actors involved in the innate immune response of the brain during the development of GBS meningitis and the potential pathophysiological consequences of this response.